eTM allows you to create your own working environment related to the Studies and Centers you work on most often (by the selection window). These settings will be saved, so that each time you log on, the same familiar settings will be displayed. The system is composed of 2 modules:
- Administration Module
- Scientific and Financial Module
To give you an idea what these modules are about, you will find a short description of each module below:
Administration Module
The Administration Module is for use only by the Application Administrator or "Super User" with rights in this module.
His major using is to:
- Enter new users and grant access to eTM
- Set up the software
- Set Thesaurus values for part of the drop down lists
- Create/maintain the list of essential documents for each country
- Create/maintain the list of milestones to track
Scientific and Financial Module
The Scientific and Financial Module gives complete control of the development of the study, GCP and monitoring of patients and dropouts.
This Module allows also tracking information on contracts with investigators as well as information on payments. There are links between the Financial Data and the Scientific Data, so e.g., you can see in a Contract all procedures performed during a study as soon as their status is payable.
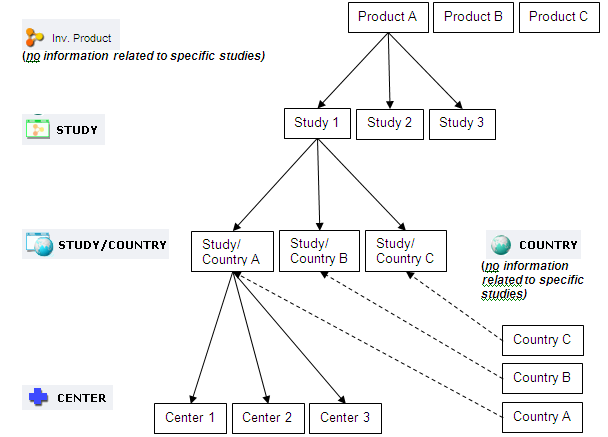
This help covers the Scientific and Financial Module. Once you log on to the Module, the selection window consisting of five levels is displayed:
- Product
- Country
- Study
- Study/Country
- Center
To facilitate the use, the help is subdivided in five chapters' analogue to the course of the data entry:
- Project Set up and Maintenance (i.e., defining projects for a product, e.g., 'Study name', and tracking of project milestones)
- Study Set up (i.e., selection of product, countries and study)
- Study Planning (i.e., setting study milestones, setting study visit schedule, setting Source Document to verify, defining personnel, selecting study/countries, planning recruitment, defining study material, entering centers, contract creation etc.)
- Study in Progress (i.e., tracking patients / visits / procedures / SDV / tracking contacts, essential documents, contract amendment, Fees etc.)
- Study Closure (i.e., closing centers, countries, study, closing contract)
Each of these main chapters will lead you step by step through all levels/screens most probably necessary at that status of the study.
For your convenience, a short overview of the information captured/available at each level is given below.
1.Investigational Product level: contains information about particular products. There is no information related to specific studies at this level.
2. Country level: contains country specific information like
- Regulatory Authority Submission
- Regulatory Authority Information
- Address Formats (only accessible for Correspondent Administrator)
There is no information related to specific studies at this level.
3. Study Level: contains all study specific information which includes:
- Study Title, Study type & phase, target number of patients/Centers
- Milestones
- Visit Schedule(s), Source Document to Verify, CRF Set
- External personnel (associated personnel) on study level
- All internal personnel implied in the study
- Contacts
- Material management
- Essential documents
- Centralized procedures
- Financial Contracts ect.
4. Study/Country Level: contains the specifics of each study/country in relation to a particular study. This includes:
- Recruitment Plan
- External (associated)/Internal personnel
- Centralized procedures
- Material management
- Essential documents
- Management of External Services
- Financial Contracts etc.
5. Center Level: tracks the creation and management of Centers within a particular study. This includes:
- Center specific external and internal personnel
- Centralized procedures
- Material management
- Essential documents
- Problem and Contact tracking
- Financial Contracts
- Patient tracking ect.
The simplified chart gives you an overview of the structure of the Scientific and Financial module.
Additionally there is a database of all external contacts regarding clinical trials which has to be maintained. All studies have external personnel associated with them (e.g., Principal Investigator, Study Nurse). eTM calls these persons 'Coontacts'.
A Contact is defined as the association of a person and a location, which allows a person to exist in more than one location. The data of the Conatacts is saved in a database independent of any trial. Provided that your role gives you access to the contact data, this database is accessible via the button 'Contact List'
How to Access to the software
How to define your Own Environment
How to Browse between the levels
How to filter
Reports
Dashboards