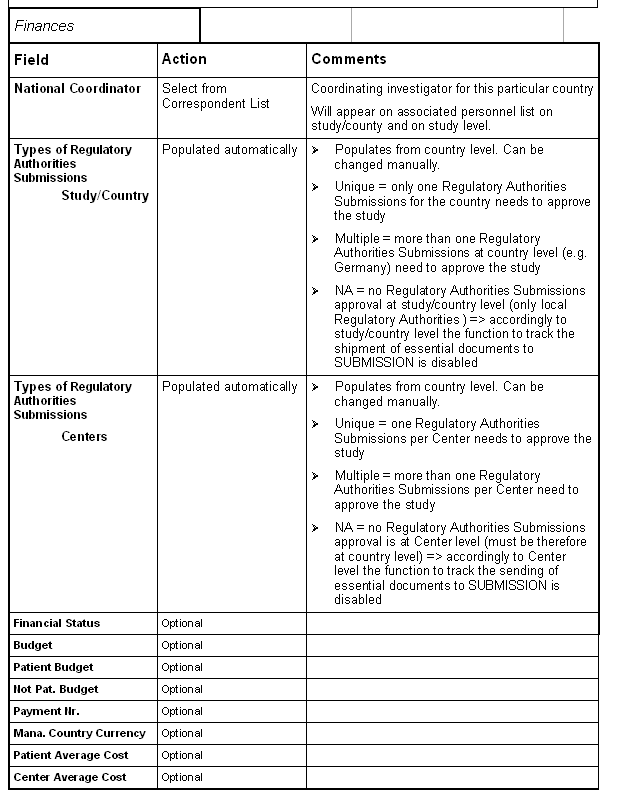
Tab: Scientific Protocol.
Study/Country Form: consists of 3 pages represented by tabs
in the screen. Each of the 3 pages can be accessed by
clicking on the corresponding tab.
Make sure that the total number of patients and Centers from all study/countries equals the target number of patients and Centers entered in Study Form. Otherwise there may be discrepancies in the reports.
Make sure that the total number of patients and Centers from all study/countries equals the target number of patients and Centers entered in Study Form. Otherwise there may be discrepancies in the reports.
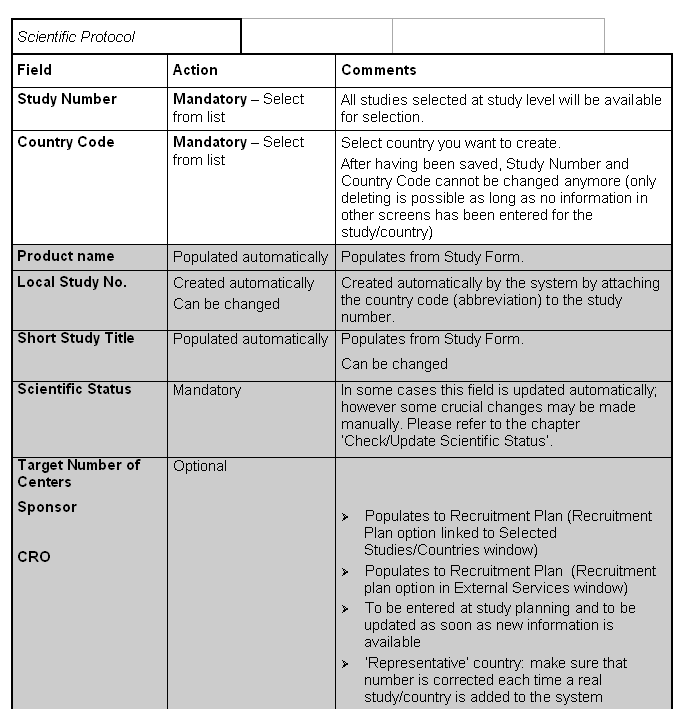

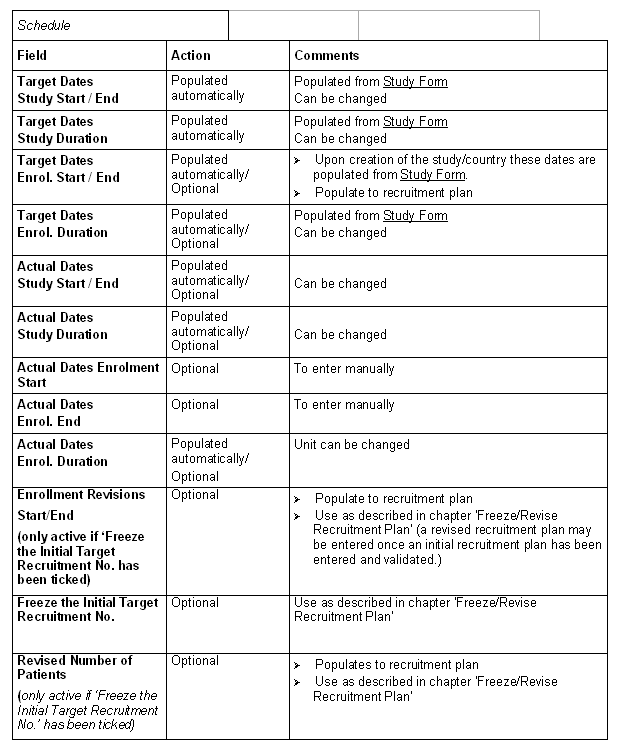
Tab: Schedule
When a study/country is created, the target enrollment dates are populated automatically from Study Form. If the dates change afterwards, they have to be changed manually in the Study/Country Form and make the necessary changes there as well).
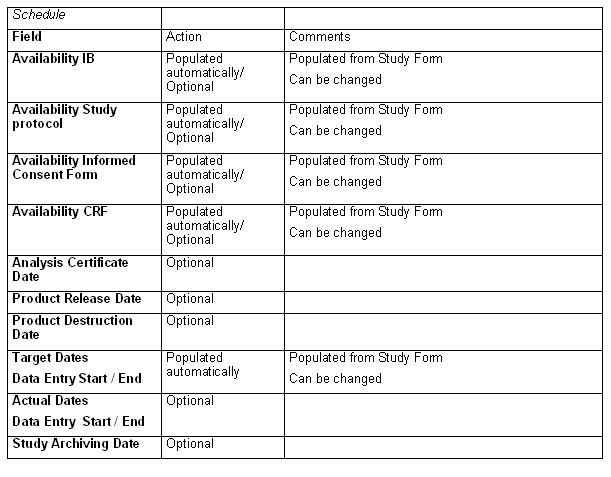
Tab: Finances
Types of Regulatory Authorities Submissions Study/Country and
Centers are populated from country level.
If for your study the types differ from that as an exception to the rule, you can change the type(s) of regulatory authorities' submissions here for your study.
If for your study the types differ from that as an exception to the rule, you can change the type(s) of regulatory authorities' submissions here for your study.