If you need additional documents
If defined as 'not unique' in the administration module, some documents can be added multiple times to the Center Investigator Essential Documents List.
Default list of essential documents displayed varies
depending on national requirements.
Essential documents which are needed at Center/investigator level for all studies are listed by default (defined as compulsory). They cannot be deleted.
Essential documents that have been defined as 'Not systematic' for Center/investigator can be added manually as required.
If defined as 'not unique' in the administration module, some documents can be added multiple times to the Center Investigator Essential Documents List.
Except for the following described columns, the others are completely variable and depending of your design (done in the administration module).
Essential documents which are needed at Center/investigator level for all studies are listed by default (defined as compulsory). They cannot be deleted.
Essential documents that have been defined as 'Not systematic' for Center/investigator can be added manually as required.
If defined as 'not unique' in the administration module, some documents can be added multiple times to the Center Investigator Essential Documents List.
Except for the following described columns, the others are completely variable and depending of your design (done in the administration module).
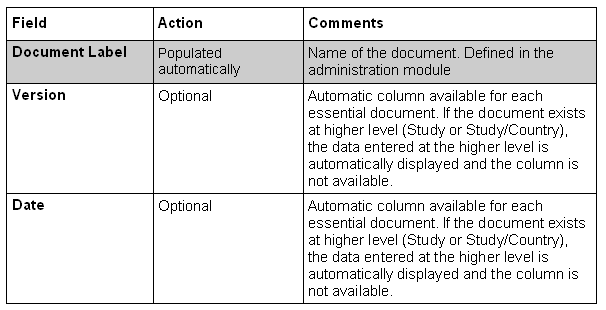
Option on Center Investigator Essential Documents List: