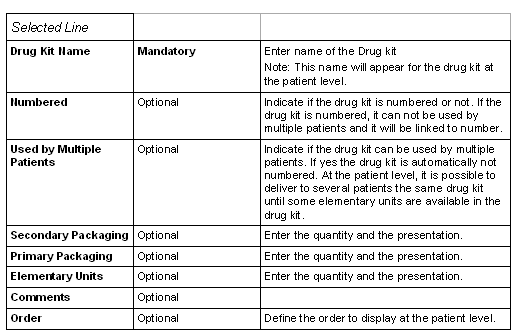
The upper part of this window allows you to
describe the drug kits.
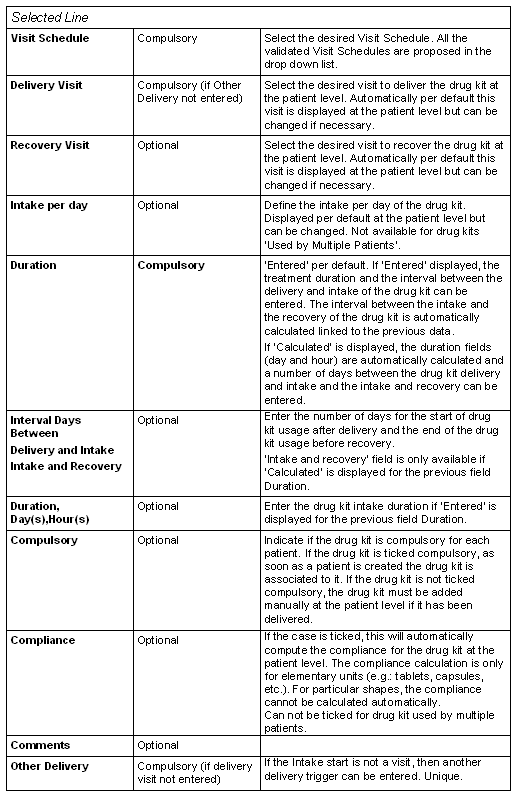
The lower part of the window allows you to specify for which visit schedule, delivered visit and recovery visit, the selected drug kit is defined.
The lower part of the window allows you to specify for which visit schedule, delivered visit and recovery visit, the selected drug kit is defined.
For each drug kit you may:
- enter a name
- define if the drug kit is numbered
- define if the drug kit can be used by multiple patients
- define the packaging (secondary, primary and elementary units)
- enter a name
- define if the drug kit is numbered
- define if the drug kit can be used by multiple patients
- define the packaging (secondary, primary and elementary units)
You can create any number of drug kits. They are described independently of each other (E.g.: Study treatment and associated treatment).
Each drug kit is described by a name and a type (numbered or not).
A drug kit is composed of secondary packaging (E.g.: boxes, etc...) containing primary packaging (E.g.: blister, etc...) containing elementary units (E.g.: tablets, etc...).
Upper Part
Lower Part